Survodutide vs Tirzepatide: How Do They Compare?
Question on this topic? Get an instant answer from August.
Survodutide and tirzepatide are both injectable medications being studied for weight loss and metabolic health. The key difference is that tirzepatide is FDA approved and widely available, while survodutide is still in clinical trials and not yet available to the public. They also work through different hormone pathways, which may matter for specific health goals.
If you are trying to understand how these two medications stack up, here is what the research shows so far.
How Does Each Medication Work?
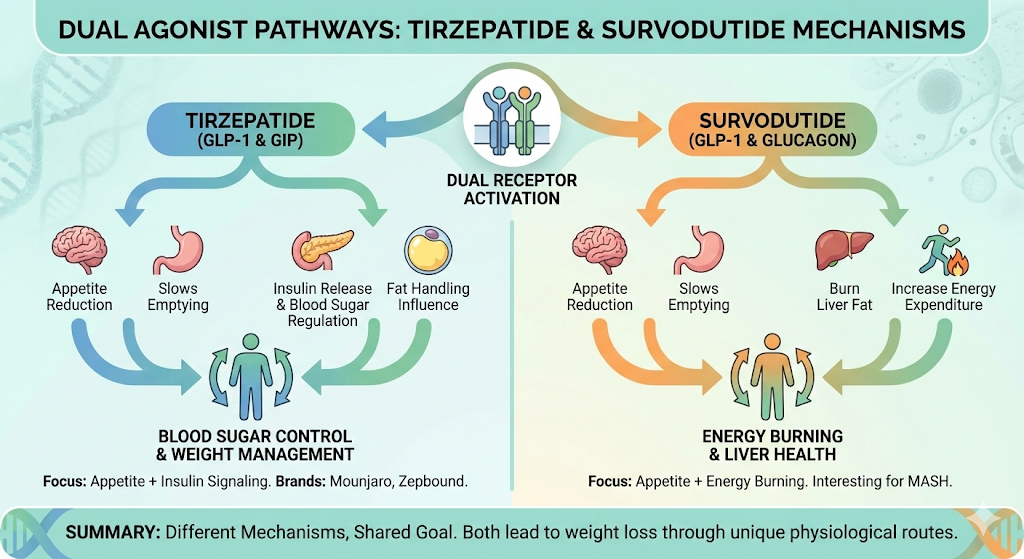
Both drugs are dual agonists, meaning they activate two hormone receptors at once. But the receptor pairs they target are different, and that changes how they affect your body.
Tirzepatide targets GLP-1 and GIP receptors. GLP-1 (glucagon-like peptide-1) reduces appetite, slows stomach emptying, and helps regulate blood sugar. GIP (glucose dependent insulinotropic polypeptide) enhances insulin release and may influence how your body handles fat. Together, these two pathways create strong effects on both blood sugar control and weight loss. Tirzepatide is sold under the brand names Mounjaro (for type 2 diabetes) and Zepbound (for weight management).
Survodutide targets GLP-1 and glucagon receptors. It shares the appetite-suppressing GLP-1 activity with tirzepatide, but instead of GIP, it activates the glucagon pathway. Glucagon helps your body increase energy expenditure and burn stored fat, particularly liver fat. This makes survodutide especially interesting for conditions like MASH (metabolic dysfunction-associated steatohepatitis), a progressive form of fatty liver disease.
So tirzepatide focuses on appetite plus insulin signaling, while survodutide focuses on appetite plus energy burning. Both lead to weight loss, but through partly different mechanisms.
What Does the Weight Loss Data Show?
This is where most people want a clear answer. Based on the clinical trial data available, tirzepatide currently shows greater weight loss results than survodutide.
A large 2022 study of 2,539 participants found that tirzepatide at doses between 5 and 15 mg produced 15 to 20.9 percent body weight loss after 72 weeks. Even at 48 weeks, participants saw roughly 14 to 19 percent weight loss. Some real-world users have reported losing over 20 percent of their body weight.
Survodutide's phase 2 obesity trial included 387 participants and showed 6.2 to 14.9 percent weight loss at the highest doses after 46 weeks. The top-end result of about 15 percent weight loss is promising, but the study was shorter and smaller than tirzepatide's pivotal trials.
It is important to note that survodutide is still in phase 3 trials. Larger, longer studies may show different numbers. But based on what we have today, tirzepatide appears to produce greater weight loss overall.
What About Blood Sugar Control?
Tirzepatide has strong data here. Clinical trials showed it reduced HbA1c (a measure of average blood sugar over three months) by up to 2.4 percentage points in people with type 2 diabetes. That is one of the largest reductions seen with any injectable diabetes medication.
Survodutide has also shown dose-dependent improvements in blood sugar levels in its phase 2 diabetes trial. However, the data set is much smaller and the results are still being confirmed in larger studies.
For people with type 2 diabetes looking for a proven option right now, tirzepatide has the stronger evidence base.
Where Does Survodutide Have an Edge?
Survodutide's unique advantage is its glucagon receptor activity, which may give it benefits that tirzepatide does not offer.
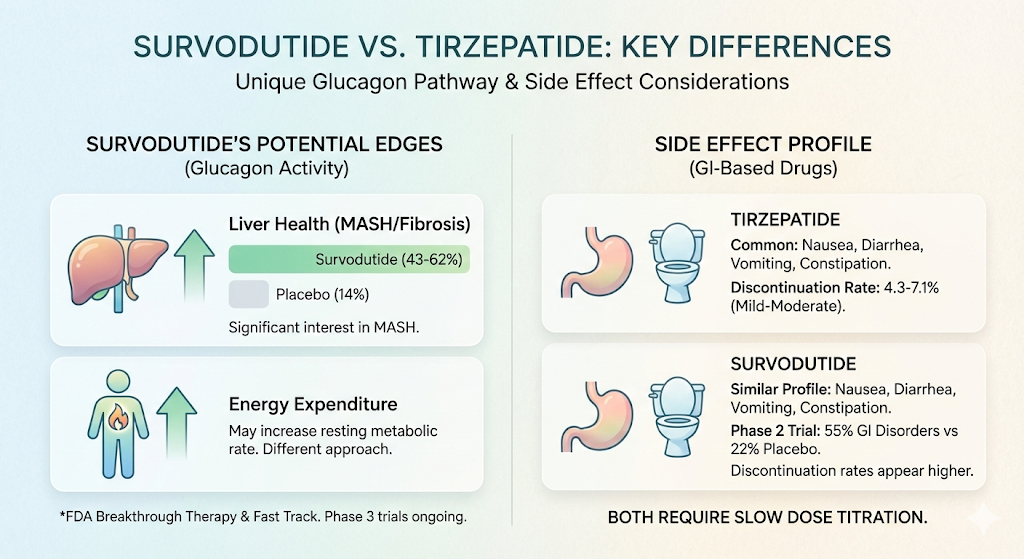
Liver health. In a phase 2 trial of people with biopsy confirmed MASH and liver fibrosis, 43 to 62 percent of survodutide treated patients met the primary liver endpoint after 48 weeks, compared to 14 percent with placebo. Between 34 and 36 percent also showed at least one stage of fibrosis improvement. These results have generated significant interest because MASH currently has very few effective treatments.
Energy expenditure. The glucagon pathway may increase resting metabolic rate, meaning your body burns more calories even at rest. This is a different approach from tirzepatide, which primarily works by reducing how much you eat.
Survodutide has received FDA Breakthrough Therapy and Fast Track designations, which signals that regulators see it as a potentially important treatment for serious conditions. Multiple phase 3 trials in both obesity and MASH are currently underway.
How Do Side Effects Compare?
Both medications cause gastrointestinal side effects. This is common across the entire class of GLP-1 based drugs.
With tirzepatide, the most frequently reported side effects include nausea, diarrhea, vomiting, and constipation. In clinical trials, about 4.3 to 7.1 percent of participants stopped treatment because of side effects. Most GI symptoms were mild to moderate and tended to improve over time, especially with gradual dose increases.
With survodutide, the side effect profile is similar: nausea, diarrhea, vomiting, and constipation. However, in one phase 2 diabetes trial, GI disorders were reported by 55 percent of survodutide participants compared to 22 percent on placebo. Discontinuation rates due to side effects appear somewhat higher than tirzepatide's, though direct head to head comparison data does not yet exist.
Both drugs require slow dose titration (starting low and increasing gradually) to help your body adjust and minimize GI symptoms.
Which One Is Available Right Now?
Tirzepatide is FDA approved and available by prescription. It comes in pre-filled injection pens dosed weekly at 2.5 mg, 5 mg, 7.5 mg, 10 mg, 12.5 mg, and 15 mg. Insurance may cover it under specific criteria for type 2 diabetes or weight management.
Survodutide is not approved or available outside of clinical trials. There is no timeline for approval yet, as phase 3 trials are still ongoing. You cannot get it through a prescription or pharmacy at this time.
The Bottom Line
Tirzepatide is proven, available option with strong weight loss and blood sugar data. Survodutide is an exciting investigational drug that may offer unique liver and metabolic benefits through its glucagon activity. If you need treatment now, tirzepatide is clear choice. If survodutide's phase 3 results confirm its early promise, it could become an important option down the road, especially for people with fatty liver disease.
Health Companion
trusted by
6Mpeople
Get clear medical guidance
on symptoms, medications, and lab reports.